
tDCS as a treatment for depression
Transcranial Direct Current Stimulation (tDCS) is a type of neuromodulation that was developed decades ago but has generated more interest in recent years. We discuss the evidence for tDCS for depression, and cover the outcomes from some recent studies.
Table of Contents
ToggleIntroduction
What is Transcranial Direct Current Stimulation (tDCS) for depression?
tDCS is the application of a small electric current to the scalp, with the aim of affecting underlying excitability of the brain, and therefore the functioning of the brain.
A history of tDCS
In 43-48 BC Scribonius Largus used live torpedo fish (a.k.a. eyed electric ray) to treat a patient with headaches. This type of ray can generate electric currents of up to 200 volts as a defence mechanism against predators. After a short period of stupor, the patient apparently experienced pain relief.
More recently, the idea of applying electrical currents to the head to treat mental disorder goes back over 200 years. In 1804, Giovanni Aldini (who was Galvani's nephew) used direct current, applied to the scalp, to treat a small number of patients with melancholia (depression) (Priori, 2003).
Controlled studies of brain polarisation for treating depression were reported in the 1970s, with one study concluding:
"Polarization appears to be therapeutically inert." (Arfai, 1970)
Modern uses
In the last few years, tDCS was available in the form of headsets which were sold as 'gaming headsets'. It was argued that the applied current could have an effect on decision-making, reaction time, etc. Of course, these claims have not been verified using proper clinical trials.
How does it work?
Although it is proposed that an electrical current applied to the scalp can affect the underlying excitability of the brain, this hasn't been demonstrated (and is difficult to do). The reviews that exist would suggest little/ no measurable change in either neurophysiological measurements (Horvath, 2015a) or cognitive performance (Horvath, 2015b).
Evidence for benefit
Controlled trials
Until recently, most of the randomised controlled trials (RCTs):
- Were done by a small number of groups;
- Included small numbers of participants;
- Were short-term (≈ 2 weeks); and,
- Involved patients who were not treatment-refractory (≥ 2 failed trials).
Systematic reviews and meta-analyses
Any published meta-analyses (e.g. Donde et al, 2017; Palm et al, 2016; Razza et al, 2020) are based on studies that were heterogenous (i.e. were highly varied). They also suggested a minimal effect in patients with treatment-refractory depression (Palm et al, 2016).
The SELECT TDCS study
Study design
The SELECT TDCS study (Brunoni et al, 2011) was a six-week trial that randomised 120 depressed patients to:
- Sertraline + tDCS;
- Sertraline + sham tDCS;
- Placebo + tDCS;
- Placebo + sham tDCS.
The main outcome measure was the MADRS depression rating scale.
Participant characteristics
Participants had a mean (SD) age of 42 ± 12 years. Sixty-eight percent were women. Other psychiatric comorbidities included: Dysthymia (26%); Generalised Anxiety Disorder (50%); Social Phobia (12%); and Panic Disorder (14%).
Baseline score on the MADRS was 30.6 ± 6, and on the HRDS-17 (Hamilton Rating Scale for Depression) it was 21.8 ± 4. This means that participants were in the 'moderate' range of both scales.
Participants had low levels of treatment resistance. Over half (56%) had only had 0 or 1 failed trials, and only 21% had had more than two failed trials.
The total duration of the current episode was relatively short. The median duration of the index episode was only 12 weeks (range 5 - 20 weeks).
Results
At week 6 (but not weeks 2 and 4), active tDCS was superior to sham tDCS, and active tDCS + sertraline was superior to active tDCS + placebo (Brunoni et al, 2013).
In terms of cognitive outcomes, all groups showed improvement and there were unrelated to depression improvement (Brunoni et al, 2016).
The DepressionDC trial
Study design
The DepressionDC trial (Padberg et al, 2017) was multi-site, randomised-controlled trial conducted at eight hospitals in Germany. The trial took place over six weeks (daily treatment for four weeks, followed by twice-weekly treatment for two weeks). All patients had been on a stable dose of antidepressants for at least four weeks.
Patients (N=160) were randomised to:
- Active tDCS;
- Sham tDCS.
Participant characteristics
Participants in the active tDCS group had a mean ± SD age of 40.2 ± 13.6. Most (62%) participants were female. The duration of the current episode was 12 ± 14.3 months.
Most participants had low levels of treatment resistance. Only 17% had had at least two robust antidepressant trials.
Baseline MADRS score was 24.5 ± 4.6. This study used the 21-item version of the HRSD scale, and baseline scores were 22.1 +- 4.6. This is broadly equivalent to a score of 20 on the HRSD-17. Consequently, participants in the DepressionDC trial were not as unwell as those in the SELECT TDCS trial.
Outcomes
In short, there was no difference in outcomes between active and sham tDCS (Burckhardt et al, 2023). The main results are shown below.
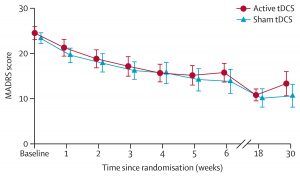
There were no differences in outcomes on secondary outcome measures (such as quality of life) or by different patient groups.
Royal College of Psychiatrists position statement
The RCPsych has published a position statement on tDCS (RCPsych, 2017):
“…tDCS may represent an effective treatment option for patients presenting with major depressive episodes”
“…tDCS offers a generally acceptable tolerability profile, which may make it a useful alternative to antidepressant medication in patients who do not wish to take medication and for those who cannot tolerate antidepressant medication.”
“…the current body of evidence does not support the use of tDCS in treatment resistant depression.”
“…the current body of evidence does not support the use of tDCS as an add-on augmentation treatment for depressed patients who are already taking an antidepressant…”
Conclusions
On the basis of published evidence, there is a lack of compelling evidence that suggests that tDCS is a useful treatment for patients with anything other than mild depression. Almost all patients in trials are relatively treatment naïve, and trials that indicate consistent benefit in those who have not responded to antidepressants are currently lacking. Whilst it has significant advantages (low risk of adverse effects), the largest and best-conducted studies show no difference between active and sham tDCS.
Like many interventions, tDCS shows a similar pattern:
- In small, open studies it shows a large effect.
- In larger randomised trials in participants with low levels of treatment resistance and severity, it shows a small-medium effect.
- In large trials with patients with longer durations of illness (and often greater severity), it shows little to no effect.
Whilst tDCS might be worth trying in patients with depression that is at the milder end of the range and/ or who struggle to tolerate antidepressants, for patients in secondary care MH services (who typically have chronic symptoms (≥ 2 years) and a history of several failed medication trials), tDCS does not appear to have a meaningful effect above sham treatment (i.e. placebo).
Patients who have access to the treatment or who can afford it should be able to try it, but there remain questions about whether it should be offered in specialist MH services.
References
PRIORI A. Brain polarization in humans: a reappraisal of an old tool for prolonged non-invasive modulation of brain excitability. Clinical Neurophysiology. 2003; 114(4): 589-595. https://doi.org/10.1016/s1388-2457(02)00437-6
ARFAI E, THEANO G, MONTAGU JD, ROBIN AA. A Controlled Study of Polarization in Depression. British Journal of Psychiatry. 1970; 116(533): 433-434. http://doi.org/10.1192/bjp.116.533.433
HORVATH JC, FORTE JD, CARTER O. Evidence that transcranial direct current stimulation (tDCS) generates little-to-no reliable neurophysiologic effect beyond MEP amplitude modulation in healthy human subjects: A systematic review. Neuropsychologia. 2015; 66: 213-236. http://doi.org/10.1016/j.neuropsychologia.2014.11.021
HORVATH JC, FORTE JD, CARTER O. Quantitative Review Finds No Evidence of Cognitive Effects in Healthy Populations From Single-session Transcranial Direct Current Stimulation (tDCS). Brain Stimulation. 2015; 8(3): 535-550. http://doi.org/10.1016/j.brs.2015.01.400
DONDÉ C, AMAD A, NIETO I, BRUNONI AR, NEUFELD NH, BELLIVIER F, POULET E, GEOFFROY P-A. Transcranial direct-current stimulation (tDCS) for bipolar depression: A systematic review and meta-analysis. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2017; 78: 123-131. https://doi.org/10.1016/j.pnpbp.2017.05.021
PALM U, HASAN A, STRUBE W, PADBERG F. tDCS for the treatment of depression: a comprehensive review. European Archives of Psychiatry and Clinical Neuroscience. 2016; 266(8): 681-694. http://dx.doi.org/10.1007/s00406-016-0674-9
RAZZA LB, PALUMBO P, MOFFA AH, CARVALHO AF, SOLMI M, LOO CK, BRUNONI AR. A systematic review and meta-analysis on the effects of transcranial direct current stimulation in depressive episodes. Depression and Anxiety. 2020; 37(7): 594-608. https://doi.org/10.1002/da.23004
BRUNONI AR, VALIENGO L, BACCARO A, ZANAO TA, DE OLIVEIRA JF, VIEIRA GP, BUENO VF, GOULART AC, BOGGIO PS, LOTUFO PA, BENSENOR IM, FREGNI F. Sertraline vs. ELectrical Current Therapy for Treating Depression Clinical Trial - SELECT TDCS: Design, rationale and objectives. Contemporary Clinical Trials. 2011; 32(1): 90-98. http://dx.doi.org/10.1016/j.cct.2010.09.007
BRUNONI AR, VALIENGO L, BACCARO A, ET AL. The Sertraline vs Electrical Current Therapy for Treating Depression Clinical Study: Results From a Factorial, Randomized, Controlled Trial. JAMA Psychiatry. 2013; 70(4): 383-391. http://doi.org/10.1001/2013.jamapsychiatry.32
BRUNONI AR, TORTELLA G, BENSEÑOR IM, LOTUFO PA, CARVALHO AF, FREGNI F. Cognitive effects of transcranial direct current stimulation in depression: Results from the SELECT-TDCS trial and insights for further clinical trials. Journal of Affective Disorders. 2016; 202: 46-52. http://dx.doi.org/10.1016/j.jad.2016.03.066
PADBERG F, KUMPF U, MANSMANN U, PALM U, PLEWNIA C, LANGGUTH B, ZWANZGER P, FALLGATTER A, NOLDEN J, BURGER M, KEESER D, RUPPRECHT R, FALKAI P, HASAN A, EGERT S, BAJBOUJ M. Prefrontal transcranial direct current stimulation (tDCS) as treatment for major depression: study design and methodology of a multicenter triple blind randomized placebo controlled trial (DepressionDC). European Archives of Psychiatry and Clinical Neuroscience. 2017; 267(8): 751-766. https://doi.org/10.1007/s00406-017-0769-y
BURKHARDT G, KUMPF U, CRISPIN A, GOERIGK S, ANDRE E, PLEWNIA C, BRENDEL B, FALLGATTER A, LANGGUTH B, ABDELNAIM M, HEBEL T, NORMANN C, FRASE L, ZWANZGER P, DIEMER J, KAMMER T, SCHÖNFELDT-LECUONA C, KAMP D, BAJBOUJ M, BEHLER N, WILKENING A, NENOV-MATT T, DECHANTSREITER E, KEESER D, BULUBAS L, PALM U, BLANKENSTEIN C, MANSMANN U, FALKAI P, BRUNONI AR, HASAN A, PADBERG F. Transcranial direct current stimulation as an additional treatment to selective serotonin reuptake inhibitors in adults with major depressive disorder in Germany (DepressionDC): a triple-blind, randomised, sham-controlled, multicentre trial. Lancet. 2023; 402(10401): 545-554. https://doi.org/10.1016/S0140-6736(23)00640-2
RCPSYCH COMMITTEE ON ECT AND RELATED TREATMENTS. Statement on Transcranial Direct Current Stimulation (tDCS) in Depression (Position statement CERT04/17). London: Royal College of Psychiatrists. http://www.rcpsych.ac.uk/pdf/Transcranial%20direct%20current%20stimulation%20-%20ECT%20ctee%20statement%20Feb%2017.pdf
Last Updated on 12 January 2025 by David Christmas
